Tutorial: Scoring with ANS and other gene signature scoring methods#
The following package contains the Python implementation of the Adjusted Neighborhood Scoring method, as well as of UCell [1], JASMINE [2] (with likelihood or odds-ratio sub-computation), the scoring approach implemented in the R package Seurat[3] proposed Tirosh et al. [4], and the two modification of it Seurat_AG and Seurat_LVG. We refer to the main article of this work for details on the scoring methods.
Content tutorial#
In this tutorial we show the usage of each available gene signature scoring methods in this package and their arguments.
Structure:
Load preprocessed data
Load cell state specific signatures
Score cell state specific signatures
Adjusted Neighborhood Scoring (ANS)
Scanpy Scoring
Seurat Scoring
Seurat_AG Scoring
Seurat_LVG Scoring
JASMINE Scoring
UCell Scoring
Visualizations
Data#
We used our preprocessed version of the PBMC dataset and the DGEX list published by Hao et al. 2021 [5]. We will use the preprocessed dataset containing B-cells, monocytes, and natural killer cells. The preprocessed dataset can be additionally downloaded manually here and the DGEX list here. The raw data can be downloaded here.
The jupyter notebook automatically downloads the preprocessed dataset and the genes sets, and places them into a ``tut_data`` folder. Manualld downloaded data should be also placed in the (newly created) tut_data foldes.
References#
[1] Andreatta, Massimo, and Santiago J. Carmona. 2021. “UCell: Robust and Scalable Single-Cell Gene Signature Scoring.” Computational and Structural Biotechnology Journal 19 (June): 3796–98.https://doi.org/10.1016/j.csbj.2021.06.043; UCell package: carmonalab/UCell
[2] Noureen, Nighat, Zhenqing Ye, Yidong Chen, Xiaojing Wang, and Siyuan Zheng. 2022. “Signature-Scoring Methods Developed for Bulk Samples Are Not Adequate for Cancer Single-Cell RNA Sequencing Data.” eLife 11 (February). https://doi.org/10.7554/eLife.71994. JASMINE R implementation: NNoureen/JASMINE
[3] AddModuleScore scoring method of R package Seurat: https://satijalab.org/seurat/reference/addmodulescore
[4] Tirosh, Itay, Benjamin Izar, Sanjay M. Prakadan, Marc H. Wadsworth 2nd, Daniel Treacy, John J. Trombetta, Asaf Rotem, et al. 2016. “Dissecting the Multicellular Ecosystem of Metastatic Melanoma by Single-Cell RNA-Seq.” Science 352 (6282): 189–96. https://doi.org/10.1126/science.aad0501
[5] Hao, Yuhan, Stephanie Hao, Erica Andersen-Nissen, William M. Mauck 3rd, Shiwei Zheng, Andrew Butler, Maddie J. Lee, et al. 2021. “Integrated Analysis of Multimodal Single-Cell Data.” Cell 184 (13): 3573–87.e29.
[1]:
import os
import warnings
warnings.simplefilter(action='ignore', category=FutureWarning)
import scanpy as sc
import pandas as pd
import gdown
from signaturescoring import score_signature
from tut_helper import get_sigs_from_DGEX_list
sc.settings.verbosity = 2
Create tutorial data folder and automatically download the data if not existant.#
[2]:
fn_adata = 'tut_data/pp_pbmc_b_mono_nk.h5ad'
fn_dgex_genes = 'tut_data/DE_by_celltype.csv'
[3]:
if not os.path.exists('./tut_data'):
os.makedirs('./tut_data')
# if tutorial data was not downloaded before, it is downloaded
if not os.path.isfile(fn_adata):
URL = 'https://drive.google.com/file/d/15DiWGfSoqtt6Fl2tK_0ik-w50rn30LQA/view?usp=drive_link'
gdown.download(URL, fn_adata, fuzzy=True)
if not os.path.isfile(fn_dgex_genes):
URL = 'https://drive.google.com/file/d/1a3Uqky2VZxCxLvGI-soCTUp3lijrfrx7/view?usp=drive_link'
gdown.download(URL, fn_dgex_genes, fuzzy=True)
Load preprocessed data#
[4]:
adata = sc.read_h5ad(fn_adata)
## To avoid errors
if 'log1p' in adata.uns_keys():
adata.uns['log1p']['base'] = None
else:
adata.uns['log1p'] = {'base': None}
The preprocessed dataset contains B-cells, Monocytes and NK-cells.
[5]:
adata.obs['celltype.l1'].value_counts()
[5]:
Mono 43553
NK 14408
B 10613
Name: celltype.l1, dtype: int64
Load cell state specific signatures#
We create celltype signatures based on the list of published differentially expressed genes per cell type. Because the cell type granularity level is lower in the DGEX genes list, we simply union the DGEX genes of all cell sub-type beloning to our types of interest, i.e., B-cells, Monocytes and NK-cells. The detailed way how to extract the signatures is implemented in the method get_sigs_from_DGEX_list of tut_helper.py.
[6]:
DE_of_celltypes = pd.read_csv(fn_dgex_genes)
[7]:
SG_subtypes = get_sigs_from_DGEX_list(adata, DE_of_celltypes, remove_overlapping=True)
Types and their subtypes:
{
"B": [
"B intermediate kappa",
"B intermediate lambda",
"B memory kappa",
"B memory lambda",
"B naive kappa",
"B naive lambda",
"Plasma",
"Plasmablast"
],
"Mono": [
"CD14 Mono",
"CD16 Mono"
],
"NK": [
"NK_1",
"NK_2",
"NK_3",
"NK_4",
"NK Proliferating",
"NK_CD56bright"
]
}
WARNING: genes are not in var_names and ignored: ['ABCB9', 'BUB1', 'CAV1', 'CHPF', 'DLGAP5', 'IGF1', 'MYO1D', 'NUGGC', 'PERP', 'UBE2C']
WARNING: genes are not in var_names and ignored: ['7-Sep', 'CDT1', 'ESCO2', 'GINS2', 'GTSE1']
[8]:
for k,v in SG_subtypes.items():
print(f'Signature for subtype {k} contains {len(v)} genes.')
Signature for subtype B contains 488 genes.
Signature for subtype Mono contains 382 genes.
Signature for subtype NK contains 243 genes.
Score cell state specific signatures#
Next we show how to score cells with each method and avaialble parameters. We will score all signatures in SG_subtypes.
All scoring methods can be called via the method score_signature of our package:
import signaturescoring as ssc
ssc.score_signature(
adata=adata,
gene_list=gene_signature,
method='[desired scoring method]',
score_name='scores',
gene_pool=None,
df_mean_var=None,
copy=False,
use_raw=False,
**kwarg # Scoring method specific keyword arguments
)
Argument |
Default |
Description |
|---|---|---|
|
required arg |
AnnData object containing the log-normalized gene expression. |
|
required arg |
A list of genes,i.e., gene expression signature, for which the cells are scored for. |
|
|
Scoring method to use. One of [‘adjusted_neighborhood_scoring’, ‘seurat_scoring’, ‘seurat_ag_scoring’,’seurat_lvg_scoring’, ‘scanpy_scoring’, ‘jasmine_scoring’, ‘ucell_scoring’] |
|
|
Column name for scores stored in |
|
|
The pool of genes out of which control genes can be selected. If it is |
|
|
A pandas DataFrame containing the average expression (and variance) for each
gene in the dataset. If |
|
False |
Indicates whether original or a copy of |
|
False |
Whether to compute gene signature score on raw data stored in |
[9]:
## Helper array to store all names of the newly created columns
all_score_names = []
Adjusted Neighborhood Scoring (ANS)#
Our proposed method.
Method specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
100 |
The number of control genes selected for each gene in the gene_list. |
|
True |
If true, the scoring method removes genes from the |
For more details, see the implementation in signaturescoring/scoring_methods/adjusted_neighborhood_scoring.py
[10]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'ANS_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with ANS
score_signature(method='adjusted_neighborhood_scoring',
adata=adata,
gene_list=gene_list,
ctrl_size=100,
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'ANS_B'
computing score 'ANS_B'
finished (0:00:04)
Scoring for gene type and storing scores in 'ANS_Mono'
computing score 'ANS_Mono'
finished (0:00:02)
Scoring for gene type and storing scores in 'ANS_NK'
computing score 'ANS_NK'
finished (0:00:03)
Scanpy scoring#
Selecting method='scanpy_scoring' will call the original score_genes method of the Scanpy package. See details on the method here.
[11]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Scanpy_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Scanpy
score_signature(method='scanpy_scoring',
adata=adata,
gene_list=gene_list,
ctrl_size=100,
n_bins=25,
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Scanpy_B'
computing score 'Scanpy_B'
finished (0:00:01)
Scoring for gene type and storing scores in 'Scanpy_Mono'
computing score 'Scanpy_Mono'
finished (0:00:01)
Scoring for gene type and storing scores in 'Scanpy_NK'
computing score 'Scanpy_NK'
finished (0:00:01)
Seurat#
The following three chapters show the usage of the scoring method implemented in the R Package `Seurat <https://satijalab.org/seurat/reference/addmodulescore>`__ and first described by Tirosh et al. (2016)and the two modifications of it.
Method specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
100 |
The number of control genes selected for each gene in the gene_list. |
|
25 |
The number of average gene expression bins to use. |
|
|
Seed for random state. If |
For more details, see the implementation in signaturescoring/scoring_methods/seurat_scoring.py
[12]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Seurat_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Seurat
score_signature(method='seurat_scoring',
adata=adata,
gene_list=gene_list,
ctrl_size=100,
n_bins=25,
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Seurat_B'
computing score 'Seurat_B'
finished (0:00:02)
Scoring for gene type and storing scores in 'Seurat_Mono'
computing score 'Seurat_Mono'
finished (0:00:01)
Scoring for gene type and storing scores in 'Seurat_NK'
computing score 'Seurat_NK'
finished (0:00:01)
Seurat_AG#
Seurat_AG is a modification of the Seurat method and uses all non-signature genes of an expression bin as control. Thus does not require the ctrl_size argunment and results in the same control set for all signature genes landing in the same expression bin.
Method specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
25 |
The number of average gene expression bins to use. |
For more details, see the implementation in signaturescoring/scoring_methods/seurat_ag_scoring.py
[13]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Seurat_AG_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Seurat_AG
score_signature(method='seurat_ag_scoring',
adata=adata,
gene_list=gene_list,
n_bins=25,
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Seurat_AG_B'
computing score 'Seurat_AG_B'
finished (0:00:04)
Scoring for gene type and storing scores in 'Seurat_AG_Mono'
computing score 'Seurat_AG_Mono'
finished (0:00:02)
Scoring for gene type and storing scores in 'Seurat_AG_NK'
computing score 'Seurat_AG_NK'
finished (0:00:04)
Seurat_LVG#
Seurat_LVG is a modification of the Seurat method and uses ctrl_size genes per expression bin with smallest variance as control. all non-signature genes of an expression bin as control. Thus resulting in the same control set for all signature genes landing in the same expression bin. The method makes use of the highly_variable_genes method provided by Scanpy. Find more details on the Scanpy method
here.
Method specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
100 |
The number of control genes selected for each gene in the gene_list. |
|
25 |
The number of average gene expression bins to use. |
|
|
The version of the least variable genes selection defines if the genes with the smallest dispersion are chosen directly from an expression bin (v1) or whether the expressions are binned a second round (v2). |
|
|
Indicates which method should be used to compute the least variable genes. We
can use |
|
5 |
If |
For more details, see the implementation in signaturescoring/scoring_methods/seurat_lvg_scoring.py
[14]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Seurat_LVG_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Seurat_LVG
score_signature(method='seurat_lvg_scoring',
adata=adata,
gene_list=gene_list,
ctrl_size=100,
n_bins=25,
lvg_computation_version="v1",
lvg_computation_method="seurat",
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Seurat_LVG_B'
computing score 'Seurat_LVG_B'
extracting highly variable genes
finished (0:00:08)
finished (0:00:11)
Scoring for gene type and storing scores in 'Seurat_LVG_Mono'
computing score 'Seurat_LVG_Mono'
extracting highly variable genes
finished (0:00:08)
finished (0:00:11)
Scoring for gene type and storing scores in 'Seurat_LVG_NK'
computing score 'Seurat_LVG_NK'
extracting highly variable genes
finished (0:00:08)
finished (0:00:12)
JASMINE scoring#
We implemented the JASMINE gene signature scoring method proposed by Andreatta et al. 2021 in Python and can be used by our package. The scoring method proposes the computation of the scores via the likelihood or the odds-ratio. We refer to the original article for detailed distinction of the methods.
Methods specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
|
The method describes, which submethod of enrichment value computation should be
used: |
|
500 |
The number of cells in a processing batch. |
|
|
Seed for random state. If |
|
|
Keyword argument for parallel execution with joblib. |
For more details, see the implementation in signaturescoring/scoring_methods/jasmine_scoring.py
Jasmine_LH#
[15]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Jasmine_LH_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Jasmine_LH
score_signature(method='jasmine_scoring',
adata=adata,
gene_list=gene_list,
score_method='likelihood',
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Jasmine_LH_B'
computing score 'Jasmine_LH_B'
finished (0:00:16)
Scoring for gene type and storing scores in 'Jasmine_LH_Mono'
computing score 'Jasmine_LH_Mono'
finished (0:00:16)
Scoring for gene type and storing scores in 'Jasmine_LH_NK'
computing score 'Jasmine_LH_NK'
finished (0:00:14)
Jasmine_OR#
[16]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'Jasmine_OR_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with Jasmine_OR
score_signature(method='jasmine_scoring',
adata=adata,
gene_list=gene_list,
score_method='oddsratio',
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'Jasmine_OR_B'
computing score 'Jasmine_OR_B'
finished (0:00:15)
Scoring for gene type and storing scores in 'Jasmine_OR_Mono'
computing score 'Jasmine_OR_Mono'
finished (0:00:16)
Scoring for gene type and storing scores in 'Jasmine_OR_NK'
computing score 'Jasmine_OR_NK'
finished (0:00:15)
UCell scoring#
We implemented the UCell gene signature scoring method proposed by Noureen et al. 2022 in Python and can be used by our package. We refer to the original article for detailed distinction of the methods.
Methods specific arguments:
Argument |
Default |
Description |
|---|---|---|
|
1500 |
Cutoff for maximum rank allowed. |
|
500 |
The number of cells in a processing batch. |
|
|
Seed for random state. If |
|
|
Keyword argument for parallel execution with joblib. |
For more details, see the implementation in signaturescoring/scoring_methods/ucell_scoring.py
[17]:
for gene_type, gene_list in SG_subtypes.items():
# defining name of the scores column in .obs
score_name = f'UCell_{gene_type}'
sc.logging.info(f'Scoring for gene type and storing scores in \'{score_name}\'')
# scoring with UCell
score_signature(method='ucell_scoring',
adata=adata,
gene_list=gene_list,
maxRank= 1500,
score_name=score_name
)
all_score_names.append(score_name)
Scoring for gene type and storing scores in 'UCell_B'
computing score 'UCell_B'
finished (0:00:20)
Scoring for gene type and storing scores in 'UCell_Mono'
computing score 'UCell_Mono'
finished (0:00:20)
Scoring for gene type and storing scores in 'UCell_NK'
computing score 'UCell_NK'
finished (0:00:20)
Visualizing scores#
[18]:
sc.tl.pca(adata)
sc.pp.neighbors(adata)
sc.tl.umap(adata)
computing PCA
with n_comps=50
finished (0:00:47)
computing neighbors
using 'X_pca' with n_pcs = 50
/local/lciernik/miniconda3/envs/test_env/lib/python3.8/site-packages/tqdm/auto.py:21: TqdmWarning: IProgress not found. Please update jupyter and ipywidgets. See https://ipywidgets.readthedocs.io/en/stable/user_install.html
from .autonotebook import tqdm as notebook_tqdm
finished (0:00:22)
computing UMAP
finished (0:00:30)
[19]:
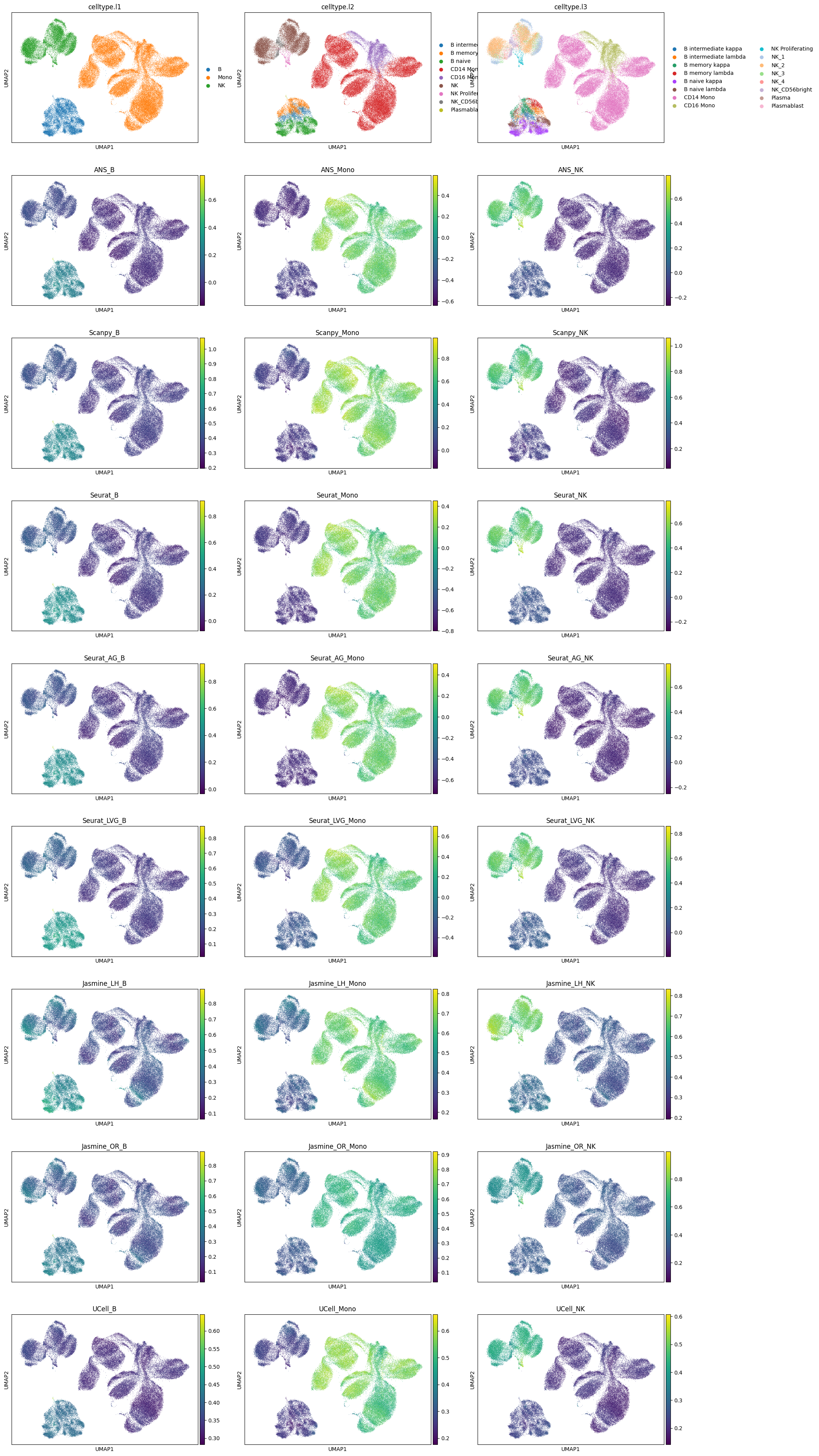
sc.pl.umap(adata, color=['celltype.l1', 'celltype.l2', 'celltype.l3']+all_score_names, ncols=3)
/local/lciernik/miniconda3/envs/test_env/lib/python3.8/site-packages/scanpy/plotting/_tools/scatterplots.py:391: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
/local/lciernik/miniconda3/envs/test_env/lib/python3.8/site-packages/scanpy/plotting/_tools/scatterplots.py:391: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
/local/lciernik/miniconda3/envs/test_env/lib/python3.8/site-packages/scanpy/plotting/_tools/scatterplots.py:391: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
Store adata for following tutorials#
[20]:
adata.write_h5ad('tut_data/pp_pbmc_b_mono_nk_scored.h5ad')